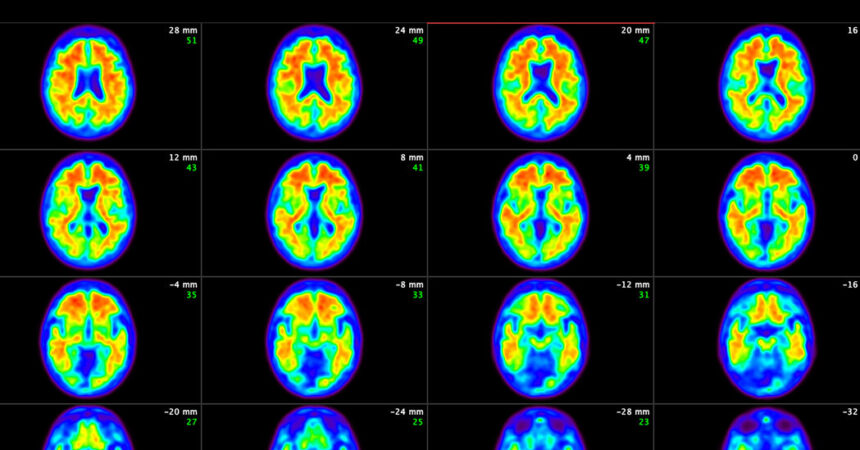
A committee of impartial advisers to the Meals and Drug Administration voted unanimously on Monday that the advantages outweigh the dangers of the latest experimental drug for Alzheimer’s illness.
Alzheimer’s afflicts greater than six million Individuals. It has no remedy, and there’s no therapy or life-style modification that may restore reminiscence loss or reverse cognitive decline.
The drug, made by Eli Lilly, is donanemab. It modestly slowed cognitive decline in sufferers within the early levels of the illness but additionally had vital security dangers, together with swelling and bleeding within the mind.
The committee concluded, although, that the results of Alzheimer’s are so dire that even a modest profit could be worthwhile.
The F.D.A. normally follows the recommendation of the company’s advisory committees however not at all times.
The drug relies on a long-held speculation that Alzheimer’s illness begins when tough onerous balls of amyloid, a protein, pile up in sufferers’ brains, adopted by a cascade of reactions resulting in the loss of life of neurons.
The concept is to deal with Alzheimer’s by attacking amyloid, clearing it from the mind. Two comparable amyloid-fighting medication had been accredited not too long ago: Leqembi, made by Eisai and Biogen, was accredited final yr. That drug’s dangers and modest advantages are much like these of donanemab. Aduhelm, made by Biogen, is the opposite drug and was accredited in 2021 however was discontinued as a result of there was inadequate proof that it may benefit sufferers.
Donanemab was anticipated to be accredited earlier this yr, however in March, the F.D.A. determined that, as an alternative, it might require donanemab to endure the scrutiny of an impartial advisory committee, a shock to Eli Lilly.
The vote, mentioned Dr. Daniel Skovronsky, chief scientific officer at Lilly, confirmed his 25-year quest to discover a solution to intervene within the Alzheimer’s illness. Now, he mentioned, the corporate is beginning a examine that, it hopes, will cease the illness earlier than signs even start.
At concern earlier than the committee on Monday had been some uncommon facets of donanemab’s medical trials, particularly that examine contributors stopped taking the drug as quickly as their amyloid was cleared. Some specialists questioned whether or not stopping was the very best technique and whether or not medical apply ought to embody halting the therapy after amyloid clearance.
Donanemab, like Leqembi, is given as intravenous infusions. Alzheimer’s specialists mentioned that the medication’ results in slowing cognitive decline are so modest that they won’t be noticeable to sufferers and households. Additionally, some famous, sufferers and households would haven’t any means of figuring out how the illness would have progressed with out the therapy.
Lilly submitted knowledge from a 76-week examine of 1,736 folks within the early levels of the illness, with both gentle cognitive impairment or gentle dementia. The contributors had been randomly assigned to get donanemab or a placebo. To measure effectiveness, the Lilly researchers assessed the sufferers’ efficiency on cognitive checks.
Cognitive decline slowed by about 4½ to 7½ months in these taking donanemab in contrast with those that bought the placebo. Almost half who took donanemab stayed on the identical cognitive stage one yr into the examine, in contrast with 29 % who bought the placebo.
However, the committee famous, practically all examine contributors had been white.
“I wish to see extra knowledge on underrepresented teams,” Colette C. Johnson, a affected person consultant on the committee, mentioned.
Three sufferers taking donanemab died with mind swelling or bleeding that was linked to the drug. The F.D.A. needed a extra detailed evaluation of the deaths of trial contributors to test for different critical security issues. Lilly complied and reported that no proof prompt extra deaths had been brought on by the drug.
Lilly’s determination to cease treating sufferers as quickly as a mind scan indicated donanemab had cleared their amyloid had actual enchantment, committee members mentioned. Sufferers might keep away from month-to-month infusions and a number of the dangers of therapy. And prices may be decrease.
In a briefing doc, Lilly prompt that persevering with the drug after amyloid is gone wouldn’t assist sufferers and may be dangerous. “As soon as the goal is cleared from the mind, continued dosing of donanemab is probably going not helpful and solely provides to therapy burden and potential dangers,” the corporate wrote.
The committee preferred the side of halting therapy however had questions.
Sarah Dolan, a panel member representing shoppers, mentioned that the potential of stopping therapy “might truly be a motivational issue for sufferers to remain compliant.” However, she mentioned, “there’ll at all times be a priority behind their head: Is it coming again? Am I getting worse?”
Dr. Constantino Iadecola of Weill Cornell Medication famous that it was not clear learn how to monitor sufferers after they cease taking the drug. “Monitoring goes to be mandatory,” he mentioned. And, he added, “how quickly will it’s important to intervene when you’ve got a sign of amyloid going up?”
Lilly scientists have estimated it might take practically 4 years for amyloid ranges to bump up over the brink once more.
One other uncommon characteristic concerned the corporate’s determination to scan sufferers’ brains for tau, a tangled spaghetti-like protein that seems in brains after amyloid accumulates. The extra tau, the more serious the cognitive decline.
Trial contributors with intermediate tau ranges — indicating an earlier stage of the illness — declined extra slowly on donanemab than these whose ranges had been excessive — supporting a widespread idea that treating sufferers as early as doable supplies a greater probability of slowing signs.
That raised a query of whether or not sufferers ought to have tau mind scans earlier than beginning the drug.
In its briefing doc, Lilly mentioned it was not recommending that tau scanning be required. “The measurement of tau ranges will not be standardized and due to this fact couldn’t be readily applied in routine medical apply,” the corporate mentioned. The F.D.A., in its evaluate, mentioned that based mostly on the proof to this point, there didn’t appear to be a purpose for sufferers to be examined for tau earlier than receiving donanemab.
Committee members had the identical response.
“From a sensible perspective I believe this might not be a clever factor to have as a barrier,” Dr. Kathleen L. Poston, a neurology professor at Stanford, mentioned.
Ultimately, these medication could also be only a foothold within the seek for an efficient therapy. However, because the committee heard, for sufferers and their households, the potential of slowing the progress of Alzheimer’s, even by a number of months, could be tantalizing.
“There’s a big unmet want right here,” mentioned Ms. Dolan, the panel’s shopper consultant.